CLINICAL TRIALS
Track intervention outcomes for patients in various research and medical settings
Disclaimer: InBody devices should be used as an adjunct tool for clinical decision-making and are not intended to diagnose or treat any diseases.
What is a clinical trial?
Clinical trials are research studies that examine a new test, procedure, or treatment and their effects on health outcomes with human subjects. People volunteer to participate in a clinical trial, which can include many types of medical interventions, such as drug and biological products, surgical or radiological procedures, devices, behavioral interventions, and disease prevention (WHO).
Clinical trials are developed by researchers and must be reviewed and approved before human subjects can start the trial. People of any age can be included in clinical trials, depending upon the intervention, device, procedure, etc. being tested (WHO).

How can body composition be used in clinical trials?
Body composition is a risk factor for poor health outcomes. Research has shown that body composition components are predictors of clinical outcomes and prognosis across multiple conditions, including cancer and stroke (Zou et al., 2023; Honma et al., 2023). Therefore, it is important to include body composition in clinical trials to fully understand the effects on human health, beyond just body weight or BMI.
In approximately 70 seconds, researchers receive the InBody Result Sheet (body composition printout) that can provide data on:
- Muscle-fat balance and visceral fat for comprehensive health risk assessment
- Fluid balance related to inflammation or underlying disease
- Nutritional status and body cell integrity
Additional features important to clinical trials are:
- Each device can store up to 100,000 tests.
- Participants can be identified by fingerprint or an ID number to easily compare tests over time.
- LB Web provides limitless storage plus connection between study sites.
Researchers can use the objective data provided by InBody devices to effectively evaluate their treatments to support patient health and wellness.
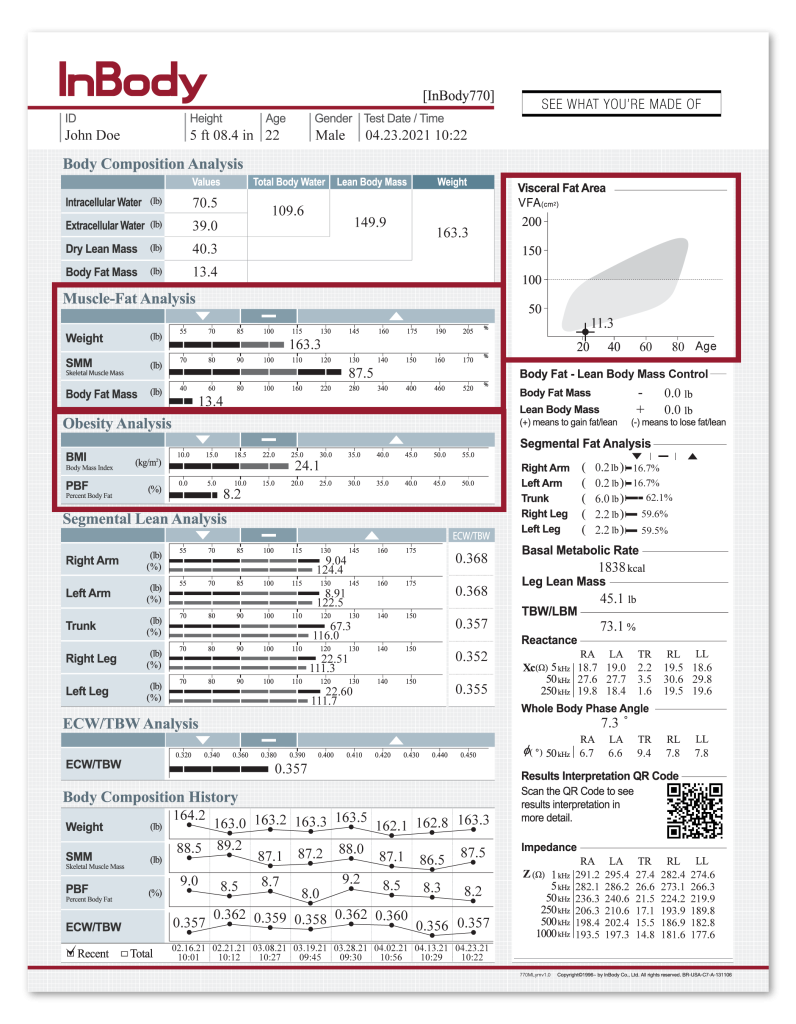
MUSCLE-FAT ANALYSIS, OBESITY ANALYSIS, AND VISCERAL FAT AREA
Assess muscle-fat balance and fat distribution to evaluate diet and exercise interventions.
Current evaluation methods that focus on body weight or BMI alone do not provide information about the physiological components of the patient, which limits our full understanding of how new treatments and procedures affect patient health. For example, Lee and colleagues (2023) found no significant difference in BMI among patients with Type 2 Diabetes across three groups in an exercise-based study but did find that the two intervention groups (virtual reality and indoor bicycle) increased muscle mass vs. the control (no exercise program) group; the intervention groups also showed lower mean blood glucose than controls following the 2-week intervention.
To provide a comprehensive assessment of their intervention, Wu and colleagues (2022) assessed fat mass, body fat percentage, and visceral fat area, in addition to body weight loss, to evaluate the outcomes and safety of a multi-phase modified ketogenic diet program. It is important to consider body composition given that obesity, visceral fat area, and sarcopenia are significantly associated with increased risk of metabolic syndrome (Kim et al., 2021).
Muscle mass can also be an important parameter to assess when determining the impact of nutritional and exercise programs. Min and colleagues (2023) found that a post-surgical inpatient exercise program increased muscle mass, promoted faster recovery, and had greater feelings of patient readiness for discharge than a usual care group. The inclusion of body composition outcomes can help determine potential mechanisms of improved outcomes and prognosis.
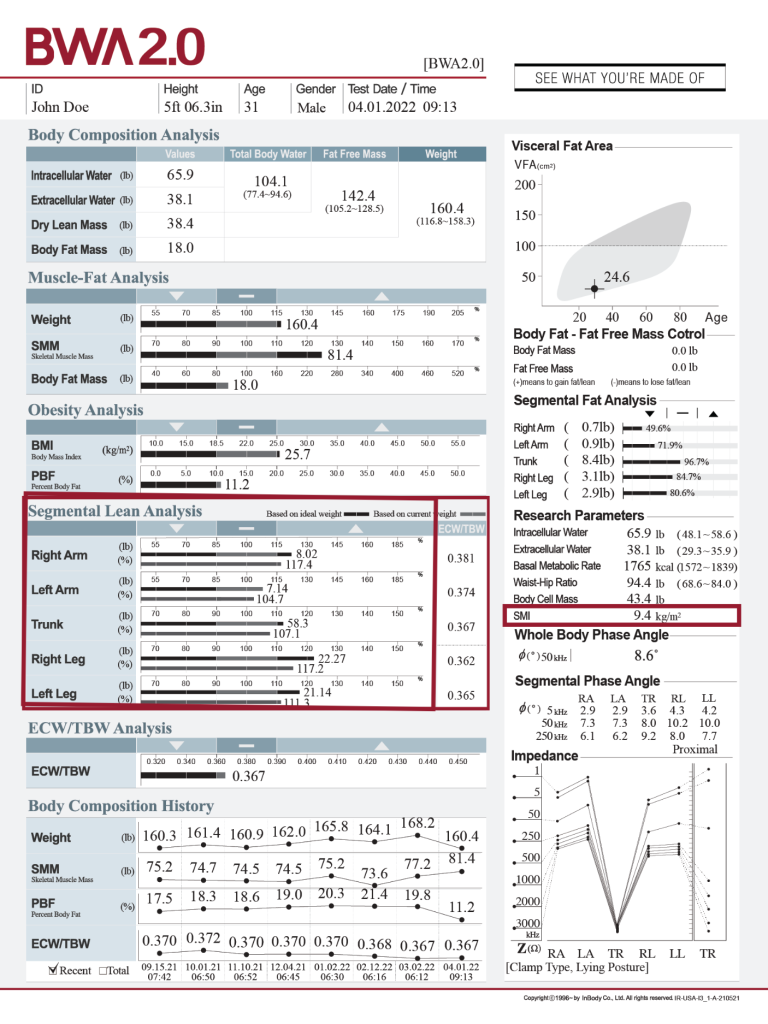
SEGMENTAL LEAN ANALYSIS
Determine muscle health and functional outcomes.
Appendicular skeletal muscle (ASM) refers to the lean mass of the arms and legs, as shown in the Segmental Lean Analysis (SLA) section of the InBody Results Sheet. ASM is used to calculate skeletal muscle mass index (SMI), a parameter used in assessing patients for sarcopenia. These values can evaluate muscle health and overall body composition in a clinical trial. The SLA section also provides segmental sufficiency percentages (i.e., the bottom bar) for each body segment to indicate if the lean body mass in that part of the body is sufficient to support the patient’s body weight. Low sufficiency percentages and/or imbalances between body segments may indicate risk for falls or injury.
Low SMI prior to esophageal cancer treatment was an independent predictor of cancer treatment-related complication (Nara et al., 2023). Clinical trials have provided evidence that interventions can improve outcomes. One trial demonstrated the safety, feasibility, and effectiveness of a supervised exercise program for advanced cancer patients which improved strength, lean body mass, skeletal muscle mass, percent body fat, quality of life, fatigue, and physical activity (Herrero et al., 2022). In addition, a 12-week circuit training exercise program among older adult women with sarcopenic obesity showed improved body composition (e.g., increased lean body mass and reduced percent body fat) in the absence of significant weight loss; the exercise group also had improved cardiovascular risk factors, in comparison to a control group (Jung et al., 2022).
BODY WATER ANALYSIS
Evaluate fluid balance, inflammation, and treatment response.
Baseline assessment and longitudinal monitoring of the extracellular water to total body water ratio (ECW/TBW) can provide important data on treatment response and possible complications. Chung and Kim (2021) found that a higher ECW/TBW ratio at day 3 post-surgery was a significant predictor of post-operative complications and in-hospital mortality. Total body water (TBW) was also assessed as a secondary outcome in a study that examined the effects of a high-intensity interval training (HIIT) program among university students with “normal weight obesity” (i.e., weight status within the normal range but high percent body fat >30%; Hu et al., 2022). They found improvements in body composition after 4-weeks of HIIT, with reduced BMI, PBF, and BFM, and increased TBW, SMM, and body cell mass.
Segmental ECW/TBW ratio can also help to assess and monitor specific parts of the body that may experience fluid retention or inflammation. These parameters are particularly useful in conditions, such as breast cancer-related lymphedema, sports injury, and physical rehabilitation. For instance, changes in body water parameters have been monitored at lymphedema onset and following surgical intervention (Yasunaga et al., 2023; Roh et al., 2023), suggesting that these parameters may be useful in evaluating response to treatment.

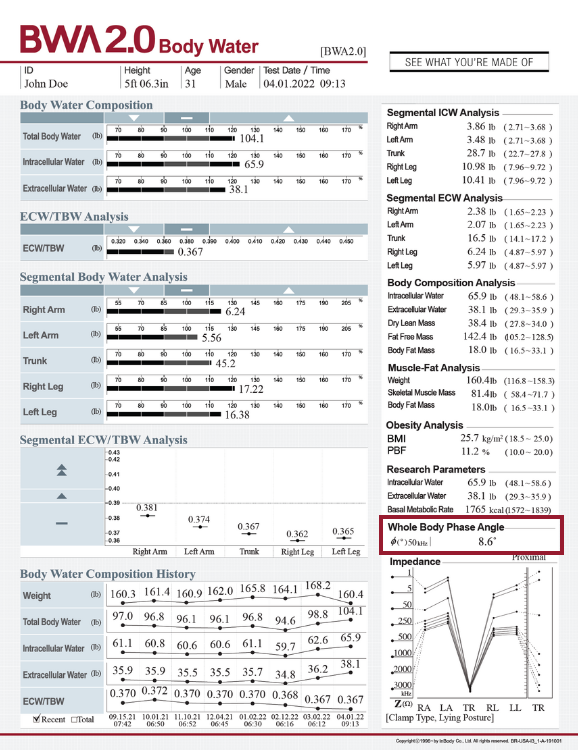
WHOLE BODY AND SEGMENTAL PHASE ANGLE
Gain insights into the effects of the intervention on cellular health.
Whole-body phase angle (PhA) provides an assessment of cellular health and nutritional status. In cases when there is fluid retention, it can cause pressure that affects cell integrity by reducing their form, structure, and stability. PhA has been associated with complications, treatment interruptions, and shorter survival among patients with head and neck cancer undergoing treatment (Yamanaka et al., 2022.), as well as an independent predictor of mortality in hemodialysis patients (Bae et al., 2022). These findings indicate the important connection between PhA and clinical outcomes.
InBody can provide objective data to track intervention effects on cell health through PhA. For example, Short and colleagues (2022)found that an exercise-based cancer rehabilitation program improved PhA among breast cancer survivors, with changes in muscle strength having a significant positive (albeit weak) relationship with changes in PhA. Segmental PhA has also been used to evaluate outcomes of surgical intervention for breast-cancer related lymphedema (Roh et al., 2023).
CLINICALTRIALS.GOV
InBody is being used to assess body composition in new and ongoing clinical trials.
Search ClinicalTrials.gov to learn more about the clinical trials that are using or have used InBody technology. Below are just a few examples:
Wearable Bioimpedance Analyzer for Tracking Body Composition Changes
In a clinic at the University of Iowa, researchers seek to determine if a wearable BIA wristband device, compared to usual care control, will improve body composition changes among patients with obesity and joint issues.
Diet and Meal Timing in Patients With Non-Alcoholic Fatty Liver Disease: A Pilot Study
A study at the Weill Medical College of Cornell University is assessing the effect of adding time-restricted eating to usual care lifestyle recommendations for diet and exercise on non-alcoholic fatty liver disease (NAFLD).
The Allegheny Singer Research Institute, in Pittsburgh, PA, aims to assess the body composition outcomes of their exercise and nutrition-based program among patients who have been treated for breast cancer.
Effects of HMB Supplementation on Recovery Following ACL Surgery
Researchers at Metabolic Technologies, Inc. and Iowa State University tested if a nutritional intervention following surgery helped patients maintain muscle mass during their recovery period.
Preventing Chemotherapy-induced Peripheral Neuropathy Using PRESIONA Exercise Program (PRESIONA)
This study, being conducted at the University of Granada (Spain), examines the effect of a therapeutic exercise program with blood flow restriction during chemotherapy treatment, as compared to usual care.
Contact Us
We’d love to hear from you.
Use the form below to send us a message!
InBody HQ
Address
625, InBody Bldg., Eonju-ro,
Gangnam-gu, Seoul 06106, Korea
Homepage
www.inbody.com
Tel
82-2-501-3939
Fax
82-2-578-5669
E-mail
info@inbody.com
InBody BWA
Address
2550 Eisenhower Avenue,
Suite C-209
Audubon, PA 19403
Homepage
www.inbodybwa.com
bwainquiries@inbody.com
InBody MEXICO
Address
Ciudad de Mexico, Mexico
Homepage
www.inbodymexico.com
Tel
55-5025-0147
Fax
–
E-mail
info.mx@inbody.com
InBody ASIA
Address
Unit 3A-11, Oval Damansara,
685 Jalan Damansara,
Kuala Lumpur 60000, Malaysia
Homepage
www.inbodyasia.com
Tel
60-3-7732-0790
Fax
–
E-mail
info@inbodyasia.com
InBody CHINA
Address
904, XingDiPlaza, No. 1698 YiShanRoad, Shanghai 201103, China
Homepage
www.inbodychina.com
Tel
86-21-6443-9705
Fax
86-21-6443-9706
E-mail
info@inbodychina.com
InBody EUROPE
Address
Gyroscoopweg 122, 1042 AZ, Amsterdam, The netherlands
Homepage
nl.inbody.com
Tel
31-20-238-6080
Fax
31-6-5734-1858
E-mail
info.eu@inbody.com
InBody JAPAN
Address
Tani Bldg., 1-28-6, Kameido, Koto-ku, Tokyo 136-0071, Japan
Homepage
www.inbody.co.jp
Tel
81-3-5875-5780
Fax
81-3-5875-5781
E-mail
inbody@inbody.co.jp
InBody INDIA
Address
Unit No. G-B 10, Ground Floor, Art Guild House, L.B.S. Marg, Kurla (West), Phoenix Market City, Mumbai 400070, India
Homepage
www.inbody.in
Tel
91-22-6223-1911
Fax
–
E-mail
india@inbody.com
Copyright© 2024 by InBody BWA. Inc. All rights reserved.
